Quantitative self-assembly prediction yields targeted nanomedicines
/Yosi Shamay, Janki Shah, Mehtap Işık, Aviram Mizrachi, Josef Leibold, Darjus F. Tschaharganeh, Daniel Roxbury, Januka Budhathoki-Uprety, Karla Nawaly, James L. Sugarman, Emily Baut, Michelle R. Neiman, Megan Dacek, Kripa S. Ganesh, Darren C. Johnson, Ramya Sridharan, Karen L. Chu, Vinagolu K. Rajasekhar, Scott W. Lowe, John D. Chodera, and Daniel A. Heller.
Nature Materials 17:361, 2018. [DOI] [PDF] [Supporting Info] [nano-drugbank]
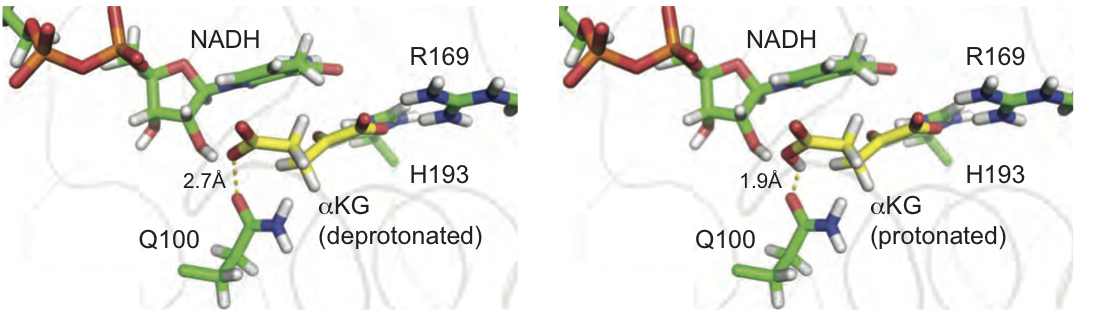
In a collaboration with the Heller Lab at MSKCC, we show how indocyanine nanoparticles can package insoluble selective kinase inhibitors with high mass loadings and efficiently deliver them to tumors.